Hsp90's conformational changes
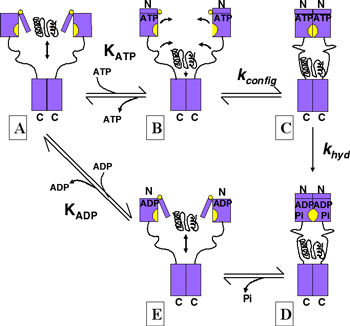
Hsp90 homodimers which are stably associated via their C-terminal domains (residues ~570-709 S. cerevisiae). During
the cycle of ATP binding and hydrolysis which is essential to the action of Hsp90 as an activator of complex assembly
it is thought that their N-terminal domains (residues 1-220 S. cerevisiae) also transiently associate. In vivo Hsp90
interacts with several co-chaperones that influence its ATPase activity and its client specificity (an up-to-date list
is maintained by Didier Picard).
We are using mass spectrometry linked with Hydrogen/Deuterium-exchange of amide backbone protons to map the conformational
changes observed when Hsp90 interacts with ligand and with various co-chaperones and client proteins. With high-resolution
structural data now available for the individual domains of Hsp90 and several of its interacting proteins, we are generating
models through assessment of their degree of structure and solvent accessibility when in specific complexes. The major advantage
of using these techniques is that we can study full-length proteins and so probe interactions between domains, whereas to-date
the majority of structural data on Hsp90 has been restricted to isolated domains due to its large size (the dimer is 170 kDa) and
degree of conformational flexibility.
Leading References
- Onuoha SC, Coulstock ET, Grossmann JG, Jackson SE (2008) "Structural studies on the co-chaperone Hop and its complexes with Hsp90." J. Mol. Biol. 379(4):732-744. PDF
- Phillips JJ, Yao ZP, Zhang W, McLaughlin S, Laue ED, Robinson CV, Jackson SE (2007) "Conformational dynamics of the molecular chaperone Hsp90 in complexes with co-chaperone and anticancer drugs." J. Mol. Biol. 372(5):1189-1203. PDF
- Caplan AJ, Jackson S, Smith D. (2003) "Hsp90 reaches new Heights." EMBO Reports 4(2):126-130. PDF
- McLaughlin SH, Jackson SE. (2002) "Folding and stability of the ligand-binding domain of the glucocorticoid receptor." Protein Sci. 11(8):1926-36. PDF
- McLaughlin SH, Smith HW, Jackson SE. (2002) "Stimulation of the weak ATPase activity of human hsp90 by a client protein." J Mol Biol. 315(4):787-98. PDF