Studies on UCH–L3 and UCH–L1
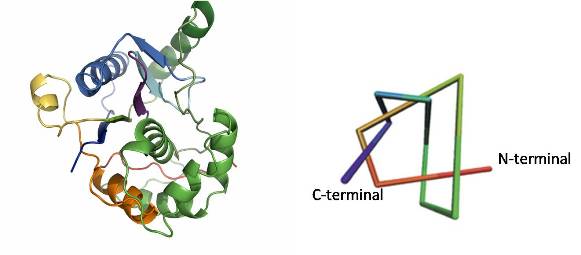
Protein folding is an extraordinary complex process that is vital for life. A new group of proteins termed "knotted" proteins that contain deep topological knots in their backbone structure has recently been discovered, adding a new and exciting twist to the protein folding problem1. The most complex knotted protein discovered to date is the human ubiquitin C–terminal hydrolase UCH–L31. The polypeptide backbone of the "penta-knotted" UCH–L3 protein contains not less than five distinct crossings and is apart from one other protein the only knotted protein found in man. Given the current lack of understanding on how knotted proteins fold and the medical importance of UCH–L3 and its structural homologue UCH–L1 (cancer and PD respectively) we are performing an in–depth folding study.
Leading References
- Andersson FI, Pina GP, Mallam AL, Blaser G, Jackson SE (2009) "Untangling the folding mechanism of the 52-knotted protein UCH-L3" FEBS J. 276:2625-2635. PDF
- Virnau P, Mirny LA, Kardar M. (2006) "Intricate Knots in Proteins: Function and Evolution" PLoS Comput. Biol. 2:1074-1079