How co-operative is the folding of a large beta-barrel protein?
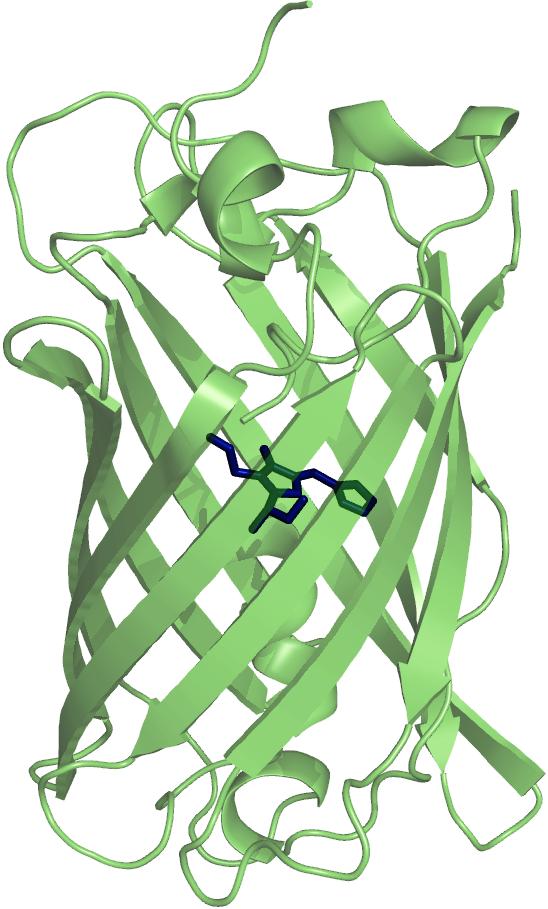
Much work has been done in characterising the folding mechanisms of many small proteins, but surprising little is currently known about the folding pathways of larger proteins. Blue Fluorescent Protein (BFP), a variant of Green Fluorescent Protein (GFP), is a 238 amino acids residue protein with a mass of 27 kDa. The protein forms an 11 stranded, β-barrel structure with a central chromophore. The chromophore is generated post-translationally by autocatalytic cyclisation of the polypeptide chain of three residues. Originating from the jellyfish Aequorea victoria, where it acts in conjuction with the photoprotein aequorin, GFP and its variants now have many uses as in vivo markers for expression, localisation and protein-protein interactions.
The presence of the chromophore affords a novel assay for complete tertiary structure formation, as when the protein is unfolded, the fluorescence is quenched. We will also use other probes for secondary and tertiary structures: Circular Dichroism and fluorescence spectroscopy using the aromatic amino acid residues of the protein and fluorescent dyes, that we engineered at various sites in the structure. We use these changes in the optical spectra upon denaturation to study the thermodynamics and kinetics of folding by standard biophysical techniques, in an effort to illucidate the folding pathway.
We are also developing novel methods to study the folding of single molecules of BFP dual-labbeled with fluorescenct dyes. In collaboration with David Klenerman, we will investigate the thermodynamics and kinetics of folding and unfolding of single molecules of BFP, utilising a nano-pipette to deliver different concentrations of denaturant. In this way, it is hoped that we will be able to measure the unfolding and folding rate constants of individual molecules.
Leading References
- Orte A, Craggs TD, White SS, Jackson SE, Klenerman D (2008) "Evidence of an Intermediate and Parallel Pathways in Protein Unfolding from Single-Molecule Fluorescence." J. Am. Chem. Soc. 130:7898-7907. PDF
- Huang JR, Craggs TD, Christodoulou J, Jackson SE (2007) "Stable intermediate states and high energy barriers in the unfolding of GFP." J. Mol. Biol. 370(2):356-371. PDF
- Khan F, Kuprov I, Craggs TD, Hore PJ, Jackson SE (2006) "19F NMR studies of the native and denatured States of green fluorescent protein. " J. Am. Chem. Soc. 128(33):10729-10737. PDF
- Jackson SE, Craggs TD, Huang JR (2006) "Understanding the folding of GFP using biophysical techniques." Expert Rev. Proteomics 3(5):545-559. PDF
- Khan F, Stott K, Jackson SE (2003) "1H, 15N and 13C backbone assignment of the green fluorescent protein (GFP). " J Biomol NMR 26(3):281-282. PDF
Current Contacts
Collaborators
- Dr David Klenerman, Dept. of Chemistry, University of Cambridge